Zokinvy adds years to the lives of young people with progeria or processing-deficient progeroid laminopathies (PDPL), empowering them to do more.1
At least 75% of patients in the United States already have substantial experience taking Zokinvy through Boston Children’s Hospital and/or the Eiger Managed Access Program, with nearly 50% of the patients taking Zokinvy in the 2 clinical studies on treatment for at least 5 years, and nearly 10% for more than 10 years.1,2
The world’s only disease-modifying treatment for progeria and PDPL3
Zokinvy is a farnesyltransferase inhibitor that prevents the accumulation of cellular progerin and progerin-like proteins. For the very first time, you can help enable patients to live longer lives.1
By inhibiting farnesylation, Zokinvy targets a key step in the pathophysiology of progeria and PDPL.1
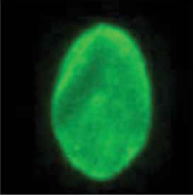
Lamin A, a normal LMNA protein product, is critical to many cellular functions.3
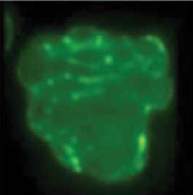
Left untreated, farnesylated protein will incorporate into the inner nuclear membranes of cells.3,4
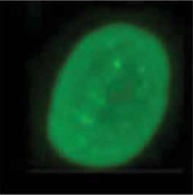
Zokinvy inhibits farnesyltransferase to prevent farnesylation and subsequent accumulation of progerin and progerin-like proteins in the inner nuclear membrane.1
Zokinvy reduces the risk of mortality, adding years to patients’ lives1
The efficacy of ZOKINVY was determined based on results from the Observational Cohort Survival Study, which retrospectively compared survival data from two phase 2 studies in patients with progeria with those from an untreated cohort.1
- The primary end point was all-cause mortality compared with historical control patient data matched by age at start of treatment, gender, and continent.1,2
- At last follow-up, treatment with Zokinvy resulted in increased mean survival of 2.5 years and a 60% reduction in risk of mortality. 1

At a 3-year follow-up, there was a 70% reduction in risk of mortality. At last follow-up, the reduction was 60%.1
-
Phase 2, open-label, single-arm trial that evaluated the efficacy of Zokinvy in 28 patients1:
- Among the 28 patients, 27 had progeria and 1 had PDPL.
- Patients received Zokinvy for 24 to 30 months.
- Patients initiated treatment with Zokinvy at 115 mg/m² twice daily.
- After 4 months of treatment, patients who tolerated treatment increased dosage to 150 mg/m² twice daily.
- Among the 28 patients treated, 27 patients with progeria were included in the survival assessment.
- The median age at treatment initiation for the 27 patients was 7.5 years (range, 3 to 16 years).
- Body weight range was 6.6 to 17.6 kg and body surface area (BSA) range was 0.38 to 0.7 m².
-
Another phase 2, open-label, single-arm trial, consisting of 2 study periods, in 26 patients1:
- In the first period, patients received Zokinvy with additional therapies for about 5 years.
- In the second period, patients received Zokinvy 150 mg/m² twice daily for up to 3 years.
- An additional 35 treatment-naïve patients with progeria enrolled in the second period of
Study 2. - The median age was 6 years (range, 2 to 17 years).
- Body weight range was 6.7 to 22.0 kg and BSA range was 0.42 to 0.90 m².
-
Although ProLon1 (Study 1) had a lower starting dosage than ProLon2 (Study 2), adverse reactions were reduced in both studies over
time.1 -
Only 4 patients (or 6.3%) withdrew from the studies due to nausea and vomiting.1
Zokinvy improves cardiovascular outcomes in patients with progeria or PDPL2,3,5
In clinical studies, Zokinvy was shown to reduce2,3,5
- Carotid artery echodensity, suggestive of a reduction in vascular wall inflammation
and fibrosis - Pulse wave velocity, a measure of arterial stiffness
Zokinvy has a well-studied safety profile1
In clinical studies, the most commonly reported adverse reactions were gastrointestinal (such as vomiting, diarrhea, and nausea).1
- The overall safety profile of Zokinvy in patients with progeria or PDPL is based on 414 patient-years of treatment exposure.
- More than 90 young people participated in clinical studies of Zokinvy; only 4 patients (6%) discontinued due to adverse reactions.2
- ~46.4% of patients with progeria have received Zokinvy for ≥5 years.1,2
- ~9.5% of patients with progeria have received Zokinvy for ≥10 years.1,2

- a Adverse reactions greater than or equal to a lower threshold of 5% are provided for greater context.
- b One patient had progeroid laminopathy with LMNA heterozygous mutation.
- c Abdominal pain includes stomach pain and abdominal pain.
- d Infection includes abdominal infection, candidiasis, chicken pox, Clostridium difficile colitis, colitis, croup, dengue fever, flu syndrome, flu-like symptoms, fungal infection, gastroenteritis, gastrointestinal infection, Helicobacter pylori infection, infection, infection viral, influenza, nail infection, otitis media, parotitis, perirectal abscess, pneumonia, small intestine infection, submandibular lymphadenitis, tonsillitis, and viral infection.
- e Upper respiratory infection includes bronchial infection, bronchitis, sinus infection, and upper respiratory infection.
- f Electrolyte abnormalities includes hypermagnesemia, hypokalemia, hyperkalemia, hyponatremia, hypercalcemia, hypokalemia, hyperphosphatemia, hypocalcemia, and hypernatremia.
- g Myelosuppression includes absolute neutrophil count decreased, low total white blood cells, lymphopenia, decreased hemoglobin, and hematocrit low.
- h Musculoskeletal pain includes arthritis, back pain, bone pain, foot pain, intercostal pain, joint pain, knee pain, leg pain, musculoskeletal pain, pain in ankle/extremity/ fingers/hip/leg/limb/lower limbs/left arm, shoulder pain, unilateral leg pain. Excluded is musculoskeletal pain for abdomen.
- i Cerebral ischemia includes central nervous system hemorrhage and ischemia cerebrovascular.
- j Ocular changes includes visual acuity change, corneal clouding, conjunctivitis, watering eyes, keratitis.
Analysis of clinical data from both studies indicates that2
- After the first 4 months of treatment, adverse reactions decreased by 70%-80%
- 94% of patients treated with Zokinvy continued beyond the initial 4-month period
- The longest treatment duration is ≥10 years

Adverse reactions, including gastrointestinal issues, can be successfully managed with supportive care and generally decrease over time.2
- Prophylactic administration of loperamide is allowed.1
- When Zokinvy is coadministered with loperamide, do not exceed loperamide 1 mg once daily when first coadministered.1
- Slowly increase loperamide dosage with caution in accordance with its approved product labeling.1
During the 4-month period after dosage increase 150 mg/m2, adverse events may be reduced by reverting to the starting dosage of 115 mg/m2 twice daily of Zokinvy.1





